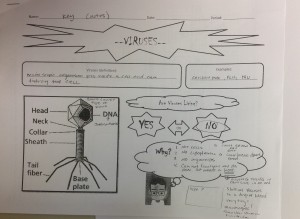
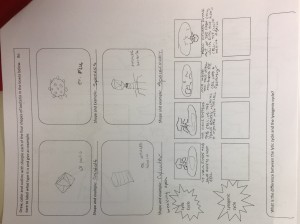
Class Notes Cornell Style
Physical and Chemical Properties of Matter
Physical Property- it is a characteristic of a substance that can be observed or measured without matter going through a chemical change, or a change in identity.
example: magazine paper.
#still remains the same
mass and weight
mass- the amount of matter in an object.
weight- is the measure of force on gravity on an object. (N) Newtons
Volume- the measure of amount of space matter occupies.
Examples ml, Cm3 1ml=1cm3
Density-physical property
d=m/v or (broken heart)
malleable- property that metals can be pounded into flat sheets. example gold ring.
conductivity- can it conduct heat? yes it’s conductive, pipe,pot, non-examples wood.
boiling point-it’s an important property of liquids.
Chemical properties- describes matter based on its ability to participate in chemical reactions and form new substance.
ex ash, gasses
ex vinegar and baking soda-reacitvity
bubbles, crystallization and gas